Thermography
Thermography, or Digital Infrared Thermal Imaging (DITI) is a non-invasive imaging procedure for monitoring physical conditions by showing thermal changes present in the body. Images can be taken of the breast, whole body, or area(s) of interest. DITI equipment is FDA Registered, painless, does not employ the use of radiation, and involves no contact with the body. Our technicians are certified through the American College of Clinical Thermology (ACCT).
Thermography can play an integral part in monitoring and maintaining health and wellbeing over a patient’s lifetime. As a non-invasive, completely safe test of physiology, Digital Infrared Thermal Imaging can provide healthcare professionals with unique findings that are adjunctive to other specialized tests that don’t show pain and dysfunction.
DITI’s major clinical value is in its high sensitivity to pathology in the vascular, muscular, neural and skeletal systems, and can contribute to the clinician’s pathogenesis and diagnosis.

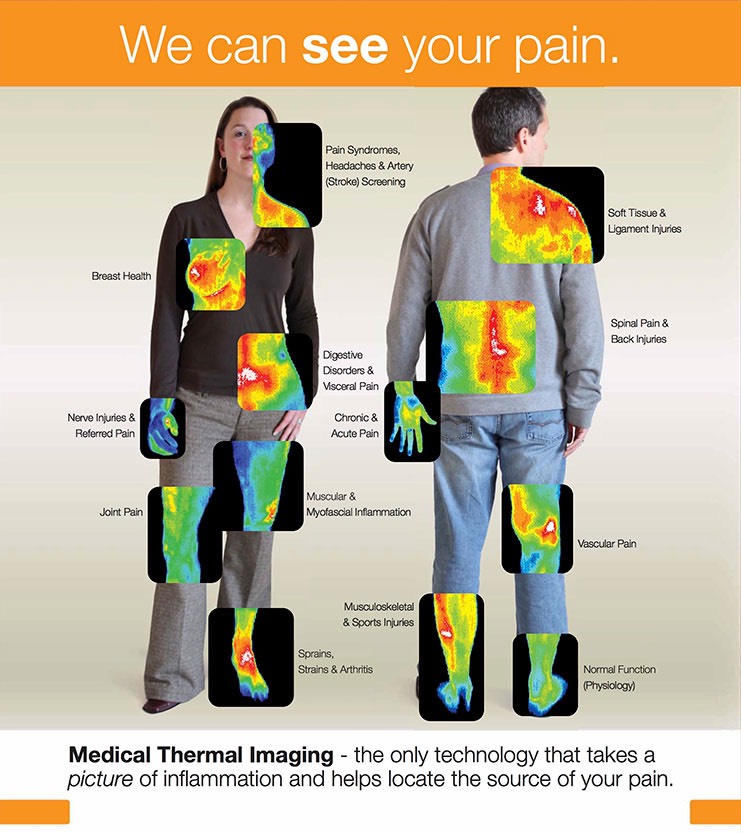
DITI PROVIDES FOR THE EARLIEST DETECTION OF PROBLEMS WITH BREAST HEALTH AS WELL AS MANY OTHER ISSUES VIA A TECHNOLOGY THAT SHOWS PHYSIOLOGY AND METABOLIC PROCESSES. X-RAYS, CT SCANS, ULTRASOUND, AND MRI ARE TECHNOLOGIES THAT SHOW STRUCTURE.
WITH DITI – AN INFRARED HEAT-DETECTING CAMERA DISPLAYS THE INFRARED ENERGY EMITTED FROM THE SURFACE OF YOUR SKIN, REFLECTED THROUGH THE SYMPATHETIC NERVOUS SYSTEM, AND CONVERTS IT INTO ELECTRICAL IMPULSES VISUALIZED IN COLOR.
Thermography is used across a wide variety of areas for evaluation and monitoring of various conditions including:
- Breast health
- Back injuries
- TMJ
- Periodontal disease
- Sinus problems
- Vascular disease
- Digestive disorders
- Nerve damage/neuropathy
- Sprains and strains
- Many other conditions
Tumors may be detected at the beginning stage of formation when vascular changes show an area is creating a new blood supply, a process known as angiogenesis. DITI can establish risk factors and you can use this information to take steps towards intervention.
Our ACCT Certified technicians can take images of the whole body or just the areas of interest. These images are stored on computer files then sent electronically to a Thermologist (a licensed Medical Doctor and Certified by the American College of Clinical Thermology (or ACCT) for interpretation and reporting. Your report is color printed and normally completed in a just a few days. We encourage you to review and address your results with your healthcare professional(s).
The FDA Classification for Our Thermography Equipment Used:
The device classification is Class I (adjunctive use) – 510(k) required
Telethermography systems are regulated as a medical device under 21 CFR 884.2980 (https://www.accessdata.fda….). The device classification is: Class I (adjunctive use) – 510(k) required
(a) Telethermographic system intended for adjunctive diagnostic screening for detection of breast cancer or other uses –(1) Identification. A telethermographic system for adjunctive diagnostic screening for detection of breast cancer or other uses is an electrically powered device with a detector that is intended to measure, without touching the patient’s skin, the self-emanating infrared radiation that reveals the temperature variations of the surface of the body. This generic type of device may include signal analysis and display equipment, patient and equipment supports, component parts, and accessories (2) Classification. Class I (general controls).
LEARN MORE ABOUT THERMOGRAPHY

JOIN THE VISTA COMMUNITY
Subscribe to our newsletter to learn more about our latest events, get healthy recipes and information to help you live a healthy lifestyle.
